shelf life calculator medical device
The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life. A plan for the storage of shelf life samples including storage conditions.

Shelf Life Calculator For Composites And Other Materials
Accelerated aging data is.

. Return to learning center. The medical device industry has long been interested in techniques for predicting the shelf life of polymer-based devices. The shelf life of medical devices is determined by putting a device through a variety of testing procedures and many engineers follow this step by procedure to avoid any.
This length of time varies depending on the type of. An accelerated ageing test for medical devices is used to simulate real time shelf-life ageing in order to validate shelf-life claims. Materials and packaging impacts the shelf life of the device.
Accelerated Aging is a process of putting packaged products into a chamber elevating the test temperature to claim a specific expiration date for a medical device product or package. Determining a medical devices shelf life the term or period during which it remains. Real-time Aging and Accelerating Aging tests are often performed together to determine the shelf life and effects of aging on materials.
Accelerated Aging Time Calculator. It is used to simulate real shelf-life aging and is conducted to validate shelf-life claims and document expiration dates. Type desired TAA TRT Values.
This process is carried out according to guidelines given in. This year that interest will increase substantially. This online service helps you to know how long your product is in good condition.
Shelf-life is the length of time you can expect a product to look and act as expected and to stay safe for use. Medical Device Shelf life. Shelf Life of Sterile Medical Devices.
Therefore the FDA requires medical device manufacturers to determine a products shelf life before sending the product to market in the United States. These accelerated tests help pinpoint possible seal and burst. Accelerated-aging tests are employed to generate this data for.
Accelerated aging testing is an FDA requirement for medical biomedical and pharmaceutical products. However sometimes it may take. Accelerated aging parameters with results supported.
Type the target shelf life Days 2. Q10 value can be changed however default is Q1020 we recommend holding this. In order to calculate expiry date you should look at Production Date on your wrapping and write it.
Sterile packaged medical devices are usually date labeled or have a pull date and may have a shelf life as defined by the 1991 FDA. This testing can identify film delineation and leaks. Establishing Shelf Life of Medical Devices.
Every medical device is required to be labeled with an expiration date that is supported by shelf-life data.

Accelerated Stability Testing And Shelf Life Calculation Pharmabeej

Nanfu 10 Pack Lr54 189 Coin Cell Batteries 1 5v Non Rechargeable Round Button Cell Batteries With Leak Proof Design 3 Year Shelf Life For Watches Clocks Calculator Sensor Medical Devices Toys Atlantic Superstore
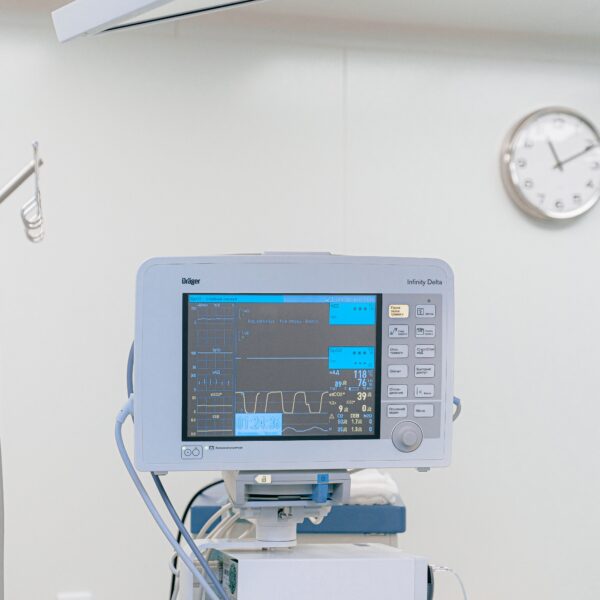
How To Determine The Shelf Life Of Medical Devices Previous Magazine

Cr2025 Primary 3v Lithium Button Cell Coin Battery For Remote Control Scales Calculator Watch Medical Instruments Computer Motherboard China Button Cell And Button Battery Price
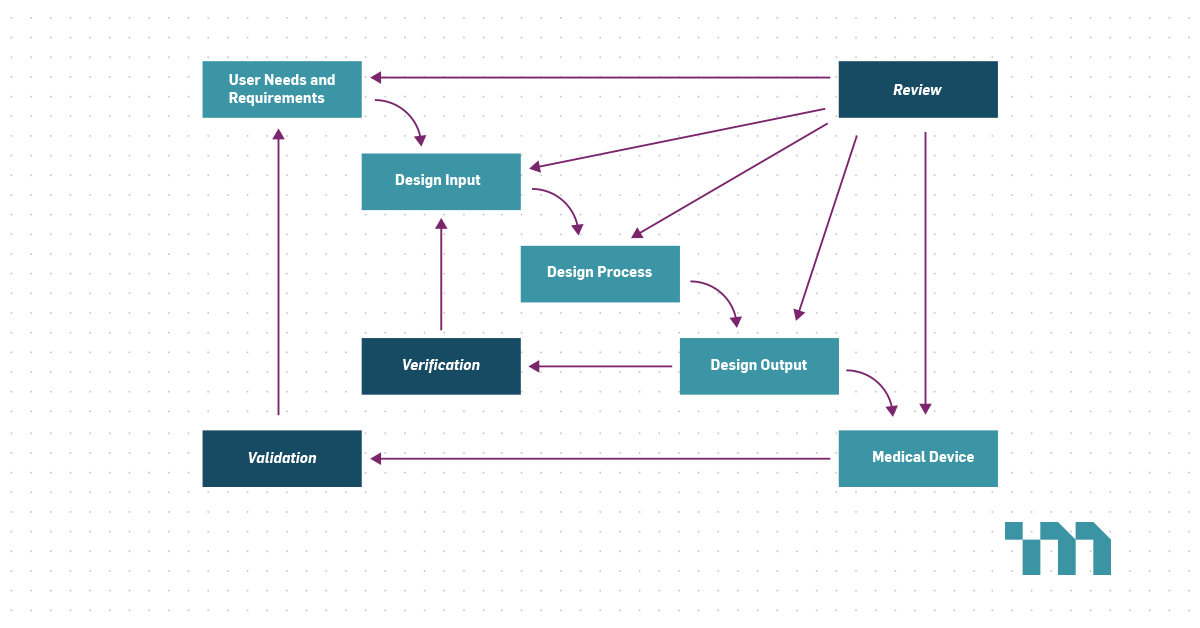
Required Verification And Validation Activities For A Fih Study

Calculation Of Expiry Date Shelf Life By Accelerated Stability Study Method In Hindi Youtube

Amazon Com Rayovac Lithium Coin Cell Battery 3 Volt Delivering Long Lasting And Continuous Power Great For Medical Devices Calculators Small Electronics Pack Of 4 Cr2016 Health Household

Accelerated Aging Calculator Medical Devices Package Testing Aat Calculator
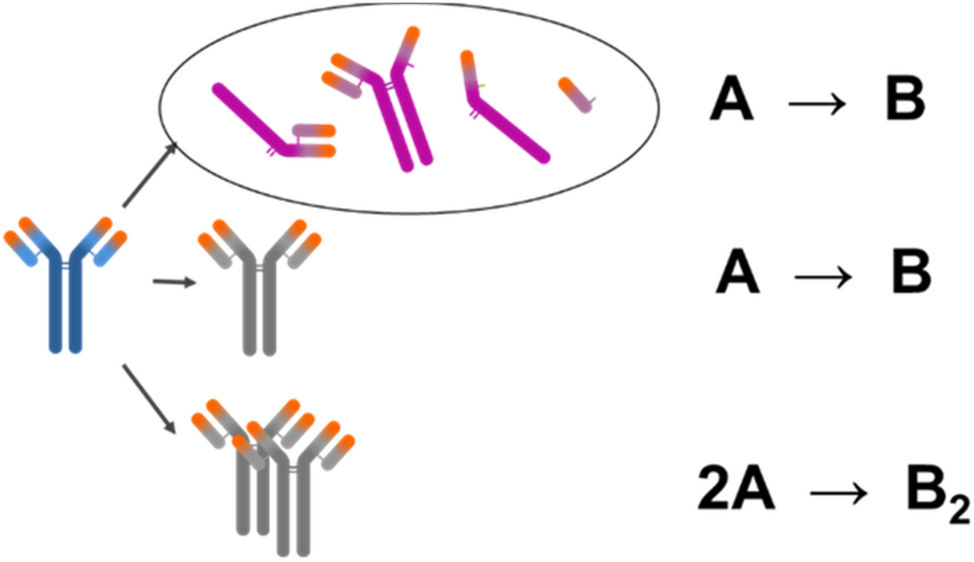
Long Term Stability Predictions Of Therapeutic Monoclonal Antibodies In Solution Using Arrhenius Based Kinetics Scientific Reports

Medical Package Testing Lab Iso 17025 Certified Packaging Compliance Labs
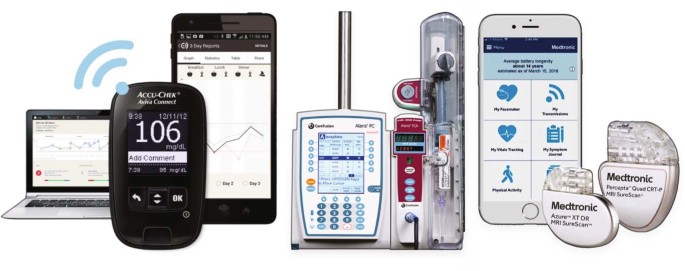
Challenges And Opportunities In Software Driven Medical Devices Nature Biomedical Engineering
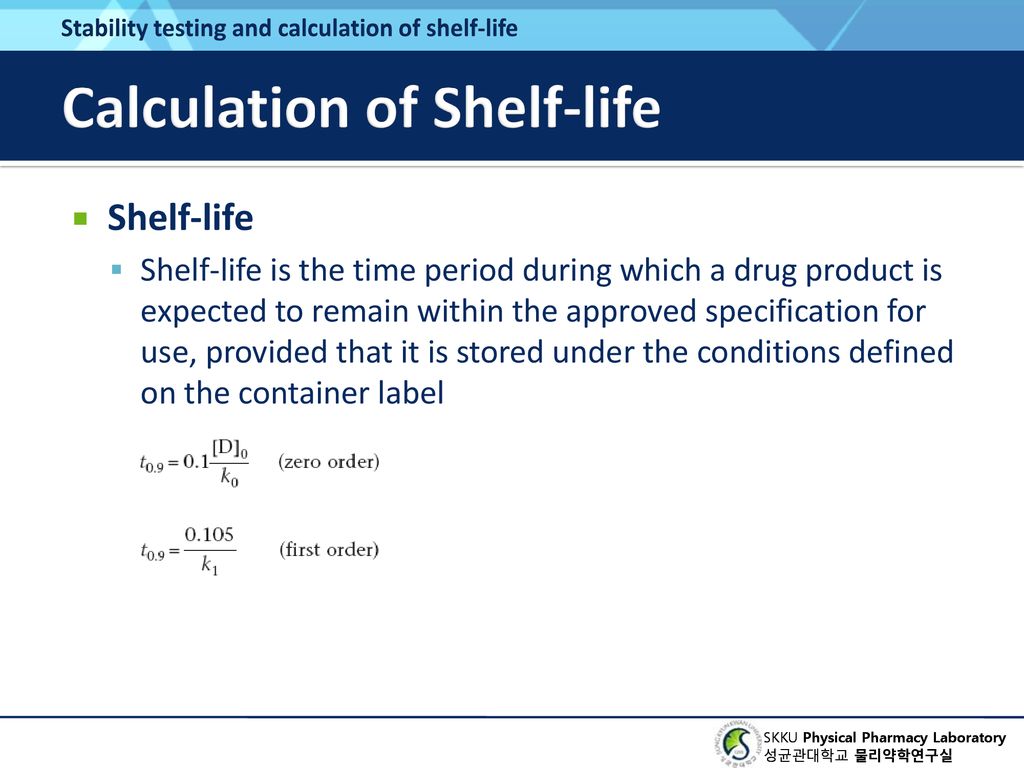
Chapter 14 Chemical Kinetics And Stability Ppt Download

Ba Ii Plus Advance Financial Calculator Dark Gray Walmart Com

Shelf Life Calculator For Composites And Other Materials
Stability Testing Life Science Outsourcing Inc
Stability Testing Life Science Outsourcing Inc

The Development Commercialization And Clinical Context Of Yttrium 90 Radiolabeled Resin And Glass Microspheres Advances In Radiation Oncology

